EM RADIATION, PHOTOELECTRIC EFFECT, X-RAYS, CRO
Electromagnetic (EM) Radiation
Electromagnetic (EM) waves are formed by varying electric and magnetic fields at right angles with each other. They are transverse in nature in that the direction of propagation is at right angles with the direction of variation of the electric and magnetic waves.
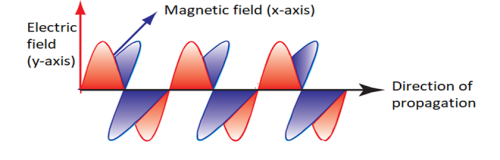
EM waves have a dual nature which means that they behave as waves as well as particles. The wave nature is used to explain characteristics such as reflection, refraction and interference. EM waves obey the wave equation;

Where c is the velocity of the EM waves in space, λ is the wavelength and f is the frequency. All EM waves travel at the same velocity (c) in space (vacuum, air) with c = 3 × 108 m/s. The wave nature of EM radiation is used to explain EM characteristics such as reflection, refraction and interference.
According to the particle theory, EM radiation consists of particles called photons, and each photon carries a discrete amount of energy E which is directly proportional to frequency f, i.e.


Combing the wave equation and photon equation leads to;

Some EM characteristics that can only be explained by the particle nature of light include photo-electric effect photoelectric effect and black body radiation.
EM radiation is categorised in terms of energy and presented in the electromagnetic spectrum in the order of increasing energy;

Electromagnetic radiation is broadly classified as either non-ionizing or ionizing radiation. UV radiation, which is made up of three energy bands, UVA (next to visible light), UVC (next to X-rays) and UVC (between UVA and UVC), acts as a bridge between non-ionizing and ionizing radiation. UVA is non-ionizing and so is the EM left to the left (light, IR, microwaves and radio waves). UVC is ionizing and so is the EM radiation to the right (X-rays, gamma rays). Ionizing radiation is energetic enough to dislodge an electron from an atom while non-ionizing radiation cannot. High energy UV, X-rays and gamma rays being ionizing radiation are capable of damaging human cells. This damaging effect is positively employed in treatment of cancer by killing cancerous cells as well as killing germs (UV being less dangerous is used in salons to sterilize equipment). X-rays and gamma rays are very penetrating. Their penetrating power is used in imaging (in hospitals and industry) since they can penetrate some organs (e.g. flesh) while they can be absorbed by others (e.g. bone).
Some X-rays have relatively lower energy (soft X-rays) while others have very high energy (hard X-rays) comparable to that of gamma rays. Compared to soft X-rays, hard X-rays are extremely penetrating (some can pass through metal). Both hard X-rays and gamma rays are high energy waves; the only difference between them is how they are produced. X-rays are produced when fast-moving electrons are decelerated (for example in an X-ray tube) or change direction (for example in a cyclotron). Either way, the energy of the electrons reduces, with the difference between the initial and final energy being released as X-rays. Gamma rays on the other hand are produced when a radioactive (unstable) nucleus disintegrates (decays). Often, alpha and beta decays which transform the decaying element (parent/mother) into a new element (daughter) produce a daughter with excess energy (excited). The daughter sheds off the excess energy (de-excites) in the form of gamma rays. Emission of gamma rays however does not transform the daughter to a new element.
In air, non-ionizing radiation is more penetrating than ionizing radiation. .Radio waves and microwaves are the most penetrating in air (on account of their long wavelengths) and are also less scattered by air particles. Ass such, they can cover very long distances in air without changing direction. It is for this reason that they are used for communications (in TV, radio, mobile phones). Microwaves are also used for cooking (microwave ovens) and in industries for drying purposes (e.g., curing of leather).
Greenhouse effect: Solar energy (radiation from the sun) that reaches the earth is composed mainly of visible light that is energetic enough to penetrate materials such as glass or plastic of a greenhouse. Once inside the greenhouse, some of the radiation it is absorbed by plants, air, soil. The absorbed radiation is then re-emitted mostly in the form of the lower energy infrared. The infrared radiation is not able to pass through glass/plastic and stays trapped in the greenhouse. Infrared radiation is basically heat hence the warmth in a greenhouse.
Photo-electric effect
Electromagnetic (EM) radiation such as light and UV radiation has a dual nature in that it exists both as a wave and a particle. As a wave, it obeys the wave equation;
 (i)
(i)
where c is the velocity, ƛ the wavelength and f the frequency. The wave nature of light is used to explain properties such as reflection, refraction, interference and dispersion.
The particle nature postulates that light is made up of discrete particles called photons, and each photon carries a discrete amount of energy E given by;
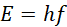 (ii)
(ii)
where h is a constant of proportionality called Planck’s constant. Particle nature of EM radiation explains properties such as photo-electric effect and black body radiation.
Photoelectic effect refers to the emission of electrons from a metal surface when light (or any other electromagnetic radiation) falls on it.
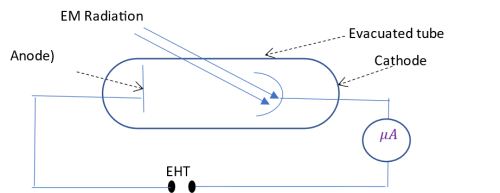
Electrons are naturally bound to the atom by electrostatic force (binding energy). When light falls on an atom, an entire photon of energy equal to or greater than the binding energy must be absorbed for the electron to be free (an atom cannot be freed by a photon of energy less than the binding energy). Part of the photon energy is used to overcome the force binding the electron to the atom (this energy is referred to as the work function, w0, (and is equal to the binding energy) while the rest is converted to kinetic energy KE of the emitted electron (photo-electron). Hence;
 (iii)
(iii)
Once emitted, the photoelectrons are attracted by the positively biased anode. The circuit is completed and the microampere records a current. (If the positive bias is increased, more electrons reach the anode hence higher current flows in the circuit).
Because the energy of a photon is dependent on the frequency, photo-electric effect cannot occur below a certain frequency. This frequency is called threshold frequency f0, (threshold frequency is the frequency below which photoelectric effect cannot occur). If for a given radiation f = f0, then the photon energy is only sufficient for removing the electron from the metal surface (no photon energy is converted to KE). Equation (iii) therefore becomes;
 (iv)
(iv)
Combining equations (iii)and (iv) gives photo-electric equation:
 (v)
(v)
Now, the wave equation is given by;
 (vi)
(vi)
When frequency equals threshold frequency f = f0 wavelength equals threshold wavelength λ = λ0. Hence;
 (viii)
(viii)
Using equations (vi) and (vii) in equation (v) leads to;
 (ix)
(ix)
It is important to note that since one photon is required to dislodge one electron, an increase in the number of photons leads to more electrons being dislodged. Increasing the intensity of light (the amount of light) increases the number of photons and consequently the number of electrons dislodged hence the current in the circuit increases
Equation (v) may be expressed as:
 (x)
(x)
Equation (x) shows that the kinetic energy of the photoelectrons depends on two factors;
- Energy of the incident photon
- Work function.
The energy needed to remove an electron from the metal surface (work function) is equal to the binding energy, no more and no less. Any excess photon energy is converted to kinetic energy. A photon of higher energy (or higher frequency since E = hf) will therefore produce photoelectrons with higher kinetic energy.
Also, for some metals, electrons are more tightly bound to the atom than in other metals. Since work function is equal to the binding energy, the lower the work function, the more the KE of the photoelectrons.
Stopping potential and maximum kinetic energy
To determine the maximum kinetic energy of the photoelectrons produced during photoelectric effect, the anode is given a negative voltage.
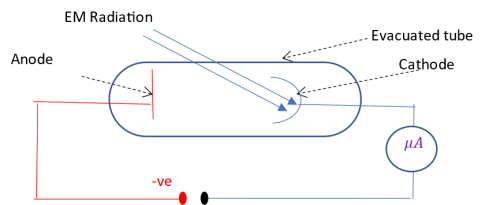
As the negative voltage is increased, the number of photoelectrons reaching the anode decreases as more electrostatic force of repulsion opposes the driving KE of the photoelectrons. Eventually, even the most energetic photoelectrons do not reach the anode. The minimum negative voltage applied to the anode that stops photoelectrons from reaching the anode is called stopping potential, Vs The stopping potential, expressed in electron-volt, eV, is thus equal to the maximum kinetic energy of the photoelectrons, i.e
 (xi)
(xi)
Where;
 (xii)
(xii)
NOTE
If the anode voltage is made more negative (less positive) the number of electrons reaching the anode gradually reduces to the point that even the most energetic electrons are repelled away from the anode. This implies that the reverse is also true: As the anode voltage becomes more positive, the number of electrons reaching the anode gradually increases to the point that even the least energetic electrons are attracted to the anode.
Since one photon is required to dislodge one electron, an increase in the number of photons leads to more electrons being dislodged hence higher current. Increasing the intensity of radiation increases the number of photons and consequently the number of electrons emitted (photoelectrons). Using light of higher intensity therefore increases the current.
During photoelectric effect some photon energy is imparted on the photoelectrons as KE;

This KE to a large extent enables the photoelectrons to move from the anode to the cathode (besides force of attraction by the anode). If the tube contained air, collision of the photoelectrons with air molecules would slow them down thereby losing some of their KE since;

Some photoelectrons will end up losing so much KE that they will not reach the anode. The magnitude of current depends on the number of electrons (Q) reaching the anode per unit time;

Fewer electrons reaching the anode would result in low current in the circuit. The tube should therefore be evacuated (air pumped out) to reduce the loss of KE of photoelectrons through collisions.
X-rays production in x-ray tube
X-rays are high-energy electromagnetic waves with energy level comparable to that of gamma rays. The only difference between the two is how they are produced. Gamma rays are produced when radioactive nuclei de-excite. X-rays on the other hand are produced when fast moving electrons are decelerated, for example by a nucleus, or when they hit a target, or when they move in a varying magnetic field (cyclotron). The kinetic energy difference is converted to X-rays.
There many ways of producing X-rays, among them production in an X-ray tube. The arrangement for production of X-rays in this manner is as shown;

The negative cathode is heated to very high temperatures leading to the emission of electrons through thermionic emission. The higher the temperature, the more the electrons emitted. The emitted electrons are accelerated towards the tungsten anode (positive) by a potential difference between the anode and the cathode. The higher the potential difference, the greater the speed of the electrons. When the electrons hit the target, their speed and hence their KE reduces. The difference between the initial and final KE is converted to X-rays.
It is important to note that increasing the cathode temperature leads to the emission of more electrons. More electrons reach the anode leading to increase in intensity (think brightness) of the X-rays produced. Also, increasing the accelerating voltage increases the kinetic energy of the electrons. The difference in KE after collision with the anode will therefore be higher. The higher the change in KE the higher the energy of the X-rays produced (more penetrating X-rays are produced).
How do X-rays produce photographs of fractures in bones? As the X-rays move through the body, they are absorbed by denser organs such as bones and transmitted by less dense organs such as flesh. The bones therefore cast a shadow on a film. If the bone has a fracture, the fractured part will not cast a shadow on the film.
A cathode ray oscilloscope (CRO)
Cathode rays refer to the stream of electrons produced through thermionic emission when a cathode is heated to a high temperature. Cathode rays are produced for example in the X-ray tube during the production of X-rays, as well as in cathode ray tube (CRT) of an oscilloscope. The difference between the two processes is that during X-ray production, the emitted electrons are accelerated to very high speeds such that when they hit the target, the loss in their kinetic energy is converted to X-rays (very energetic EM radiation). In the cathode ray tube on the other hand, the electrons are accelerated to a much lower speed such that the change in KE is converted to visible light which is a much weaker EM radiation.
A cathode ray oscilloscope (CRO) is an instrument used for the observation, measurement, and analysis of waveforms. A CRT (cathode ray tube) of a CRO has four main components:
- Electron gun
- Vertical deflection plates (Y-plates)
- Horizontal deflection plates (X-plates)
- fluorescent screen
The components are housed in a highly evacuated structure. The evacuation minimises ionisation that may lead to loss of kinetic energy of the electrons as they move from the electron gun to the screen which may affect clarity of the waveform generated on the screen.

a) The electron gun:
This is the source of a focused and accelerated electron beam. It is made up of three components:
- Heating coil (cathode)
- Control grid
- Anodes
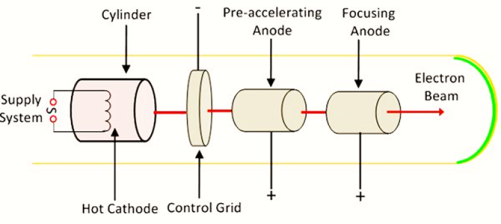
The cathode produces electrons by thermionic emission (heated). After emission, the electrons pass through the control grid. The control grid has a negative biasing. Its function is to control the intensity (number) of the transmitted electrons. The electron beam is accelerated by a highly positive pre-accelerating or accelerating anode (at about 1500 V) and directed by the focussing anode (at about 500 V). There are two ways focussing of the electron beam can be achieved;
- Electrostatic focussing which uses an electric field
- Electromagnetic focussing which employs a magnetic field.
A CRT employs an electric field for focussing electrons. A television tube however employs a magnetic field for focussing.
b) Vertical deflection plates – moves the beam vertically
c) Horizontal deflection plates – moves the plate horizontally.
NOTE: The vertical and horizontal deflection plates enable the electron beam to reach any part of the fluorescent screen.
d) Fluorescent screen/monitor - the screen is made of phosphor. When the electron beam hits the screen, the phosphor atoms are excited and light is emitted at that point. The screen therefore turns electric energy (beam of fast-moving electrons) into light energy. The bright spot on the phosphor monitor moves due to the effect of the electrostatic forces on the mutually perpendicular Y and X-plates and outlines a waveform based on the input signal.
NOTE
When the heat current is increased, the temperature of the cathode increases leading to an increase in the number of electrons emitted. The intensity of the EM radiation therefore increases. When the anode potential is increased, the kinetic energy of the electrons increases
CRO signal is a sine (cosine) curve where:


Examples


3 Comment(s)
Matosh (Sat, 31st May 2025, 6:06 PM)
the notes are impressing
Reply
Matosh (Sat, 31st May 2025, 6:06 PM)
the notes are impressing
Reply
Matosh (Sat, 31st May 2025, 6:06 PM)
the notes are impressing
Reply